Buy with Confidence with:
- Professional installation and training.
- Diverse models with modern specifications.
- Comprehensive educational support on device usage.
- A skilled and responsive maintenance team.
- Guaranteed availability of spare parts.
Device Purchase Stages:
- Pre-sale experience (Demo)
- Fast and immediate supply
- Professional staff training
- Immediate technical support and continuous communication
- Periodic software updates
Product Description
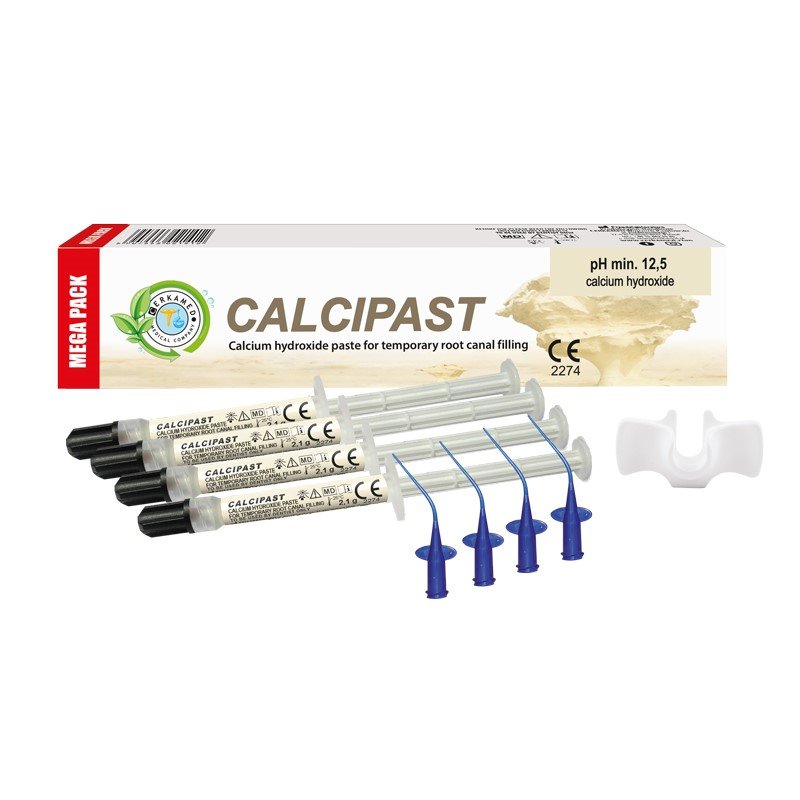
CALCIPAST Root canal filling
Composition:
- Calcium hydroxide. Hydroxyapatite. Barium sulphate. Excipients.
- Intended Use:
- Intended for use in dental treatment for temporary root canal filling. Additionally, CALCIPAST may be used for temporary filling of deep cavities during indirect or direct pulp capping.
- Mechanism of Action and Properties:
- Has a re-mineralizing effect on dental tissues thanks to its high content of calcium hydroxide. The product supports restoration of damaged periapical tissues. pH 12.5–13. Radiopaque. Non-hardening material


Instructions for Use:
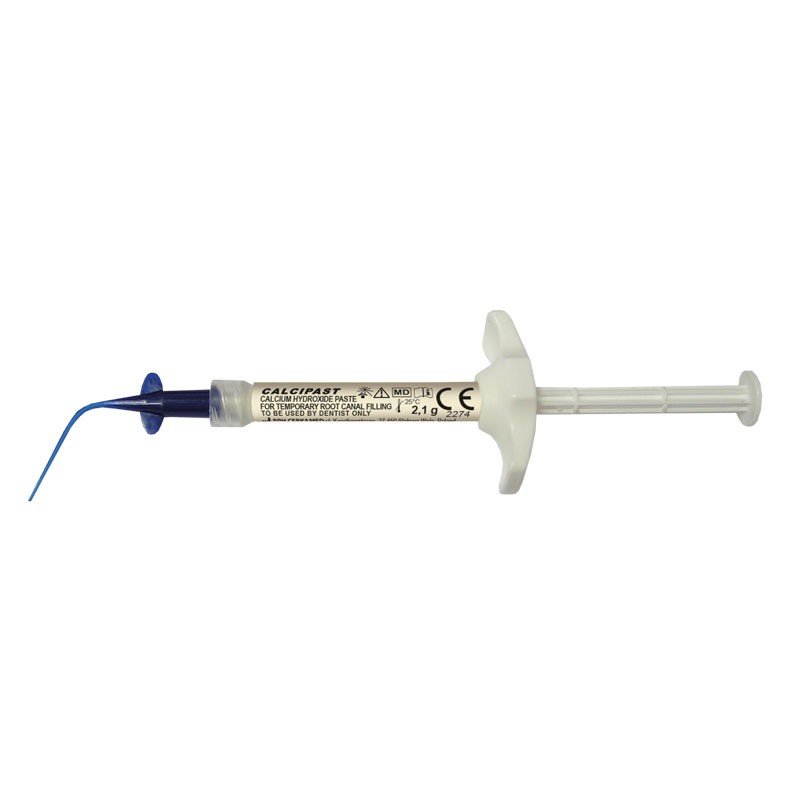
- Before treatment, dip the provided applicator in ethanol, allow it to dry, and then attach it onto the syringe.
- Before use, check if the applicator is correctly attached onto the syringe and then expel a small amount from the syringe to check the paste flow through the applicator.
- The syringe grip plate provides a comfortable grip for easy dispensing management, reducing the risk of pushing the product through the root apex and minimizing the difficulties associated with expelling the product.
- For MEGA PACK, manually put the grip plate onto the syringe before use.
- The grip plate should be put onto the capped syringe, as shown on the product packaging (a distinct "click" is heard when correctly applied).
- When used directly in the tooth canal: Measure the length of the canal, adjust the endostop on the applicator to a length 2-3 mm shorter than the measured length of the canal, dispense the paste in the canal, remove excess paste, close the cavity tightly.
- After the paste has been applied, an X-ray should be taken to check that the paste has adequately filled the canal and has not gone beyond the apex.
- When used in a cavity: Dispense the paste at the bottom of the cavity, close the cavity tightly.
- Leave the filling in the cavity or root canal for 1 week to 30 days.
- The product is intended for multiple use, while the applicator is for single use only.
- Reusing the applicator may pose a risk of secondary infection.
- The grip plate is for multiple use.
Warnings and Precautions:
- Irritant to oral mucosa and skin. Causes serious eye damage.
- In case of contact, rinse immediately with plenty of water and seek medical help.
- If ingested, do not induce vomiting. Drink plenty of water. Seek medical help.
- Use dental dam, disposable gloves, safety goggles/visors and protective clothing when handling the product.
- Endodontic treatment may involve temporary pain and postoperative tenderness.
- The paste should be carefully removed from the root canal before the final filling, as the presence of the formulation may affect the bond strength of dentin to the tooth restorative materials. The use of temporary materials may be associated with bacterial microleakage. CALCIPAST has cytotoxic potential.
- Do not expel the paste if the applicator tip is wedged inside the canal. The applicator must be loosely positioned in the canal.
- Before using CALCIPAST, the working length of the root canal should be determined.
- Excessive amounts of temporary material used to fill the root canal may lead to it being pushed beyond the canal apex. Particular care should be taken in anatomically sensitive areas such as the mandibular canal, mandibular opening or maxillary sinuses.
- Keep out of reach of children.
- Any serious incident involving the product should be reported to the manufacturer and the competent authority of the State in which the user or patient is domiciled.
- Contraindications:
- Do not use for patients who are hypersensitive to the product components.
Storage:
- Keep in original packaging below 25°C. Expiry date is placed on the main container. Once container is open, the expiry date does not change if the container is securely closed after each use.
- Handling the Product Container:
- Empty containers should be disposed of responsibly or returned to the manufacturer.
- Packaging:
- CALCIPAST: Syringe containing 2.1 g of preparation, disposable applicators (2 pieces), grip plate. CALCIPAST MEGA PACK: 4 syringes of preparation (each containing 2.1g of paste), disposable applicators (4 pieces), grip plate.
Frequently Asked Questions
Answers to your questions and inquiries about the product
What is CALCIPAST?
It is a calcium hydroxide-based paste used as a temporary filling material for root canals in dental treatment.
What are the uses of CALCIPAST?
It is primarily used for temporary filling of root canals, and can also be used for temporary filling of deep cavities.
How does CALCIPAST work?
It promotes remineralization of dental tissues and supports restoration of periapical tissues due to its high calcium hydroxide content.
Is CALCIPAST safe?
Yes, it is generally safe when used correctly. However, it should be avoided in patients with hypersensitivity to its components. It is an irritant to skin and eyes, so precautions must be taken.
How is CALCIPAST used?
It is injected into the root canal (or cavity) using the provided applicator. The canal length should be measured and the applicator adjusted before injection.
Can I reuse the applicator?
No, the applicators are for single use only.
Can I leave CALCIPAST in the canal permanently?
No, CALCIPAST is a temporary filling. It must be removed before the final root canal filling. It is typically left for 1 week to 30 days.
Is CALCIPAST radiopaque?
Yes, it is radiopaque, meaning it shows up clearly on X-rays.
What is the pH of CALCIPAST?
Its pH is 12.5-13.
Brand or manufacturer
Meet Qasr Al-Tibb Medical Partners

Eighteenth company
Eighteeth is a brand of Changzhou Sifary Medical Technology Co., Ltd., a company specializing in the manufacture of dental equipment and instruments. Founded in 2016 in Changzhou, Jiangsu, China. The company is recognized as a leader in the local dental medical equipment industry and is committed to building the leading platform for the globalization of Chinese dental instruments.
Mission and Vision
Eighteeth aims to provide high-quality dental products and services to dentists and patients around the world. The company is dedicated to innovation, quality, and global expansion, with a vision to become a leading company in the manufacture of digital solutions in the dental industry.
Products and Services
Eighteeth offers a wide range of high-quality dental products, including:
- Root Canal Treatment: Root canal preparation machines, ultrasonic scalers, and irrigation solutions.
- Imaging Systems: Cone-beam computed tomography (CBCT) scanners and intraoral scanners (such as the Helios series), and digital impression systems.
- Restoration and Aesthetic Solutions: Light-curing units, dental implants, and aesthetic restoration tools.
- Diagnostic Equipment: Apex locators, X-ray systems, and intraoral sensors.
Products are meticulously designed, incorporating advanced technologies such as artificial intelligence and 3D imaging to enhance dental workflows and patient outcomes.
Global Reach
Eighteeth products are exported to 154 countries and regions, with partnerships with over 340 prestigious universities and thousands of dental experts worldwide. The brand has a strong presence in international markets, including Europe, the Middle East, and North America.
Innovation and Research and Development
The company has a professional research and development team with over 700 employees, including senior managers with experience in leading companies such as Dentsply Sirona and Mindray. Research and development efforts at Eighteeth focus on digital dentistry and solutions that rely on artificial intelligence and intelligent manufacturing, ensuring advanced products and cost-effective solutions.
Recent Achievements
- Acquisition of Zhean Healthcare: In 2023, Eighteeth acquired Zhean Healthcare, a leading manufacturer of intraoral scanners, to expand its portfolio in digital dentistry.
- Exhibitions and Launches: Eighteeth regularly participates in global dental exhibitions, such as AEEDC Dubai and DenTech China, and presents innovative products such as the CBCT FinScan F350 and intraoral scanners from the Helios series.
Commitment to Quality
Eighteeth products have certifications from international standards, including ISO, CE, and FDA, which ensures safety, reliability, and compliance with global regulations.
Future Goals
Eighteeth plans to build a digital ecosystem for dentistry and a patient information platform to enhance communication between the dentist and the patient and simplify dental workflows. The company aims to lead the digital transformation of the dental industry.