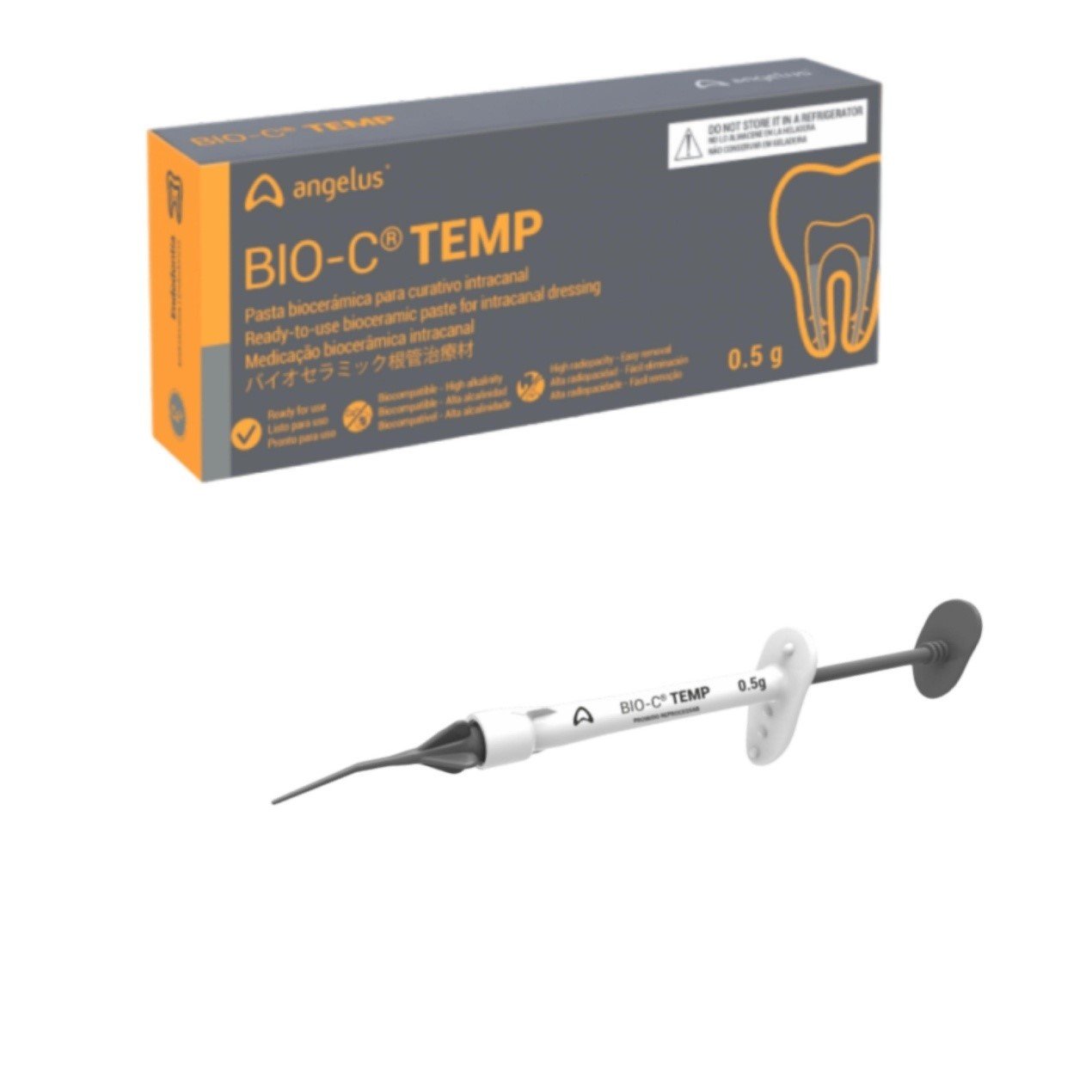
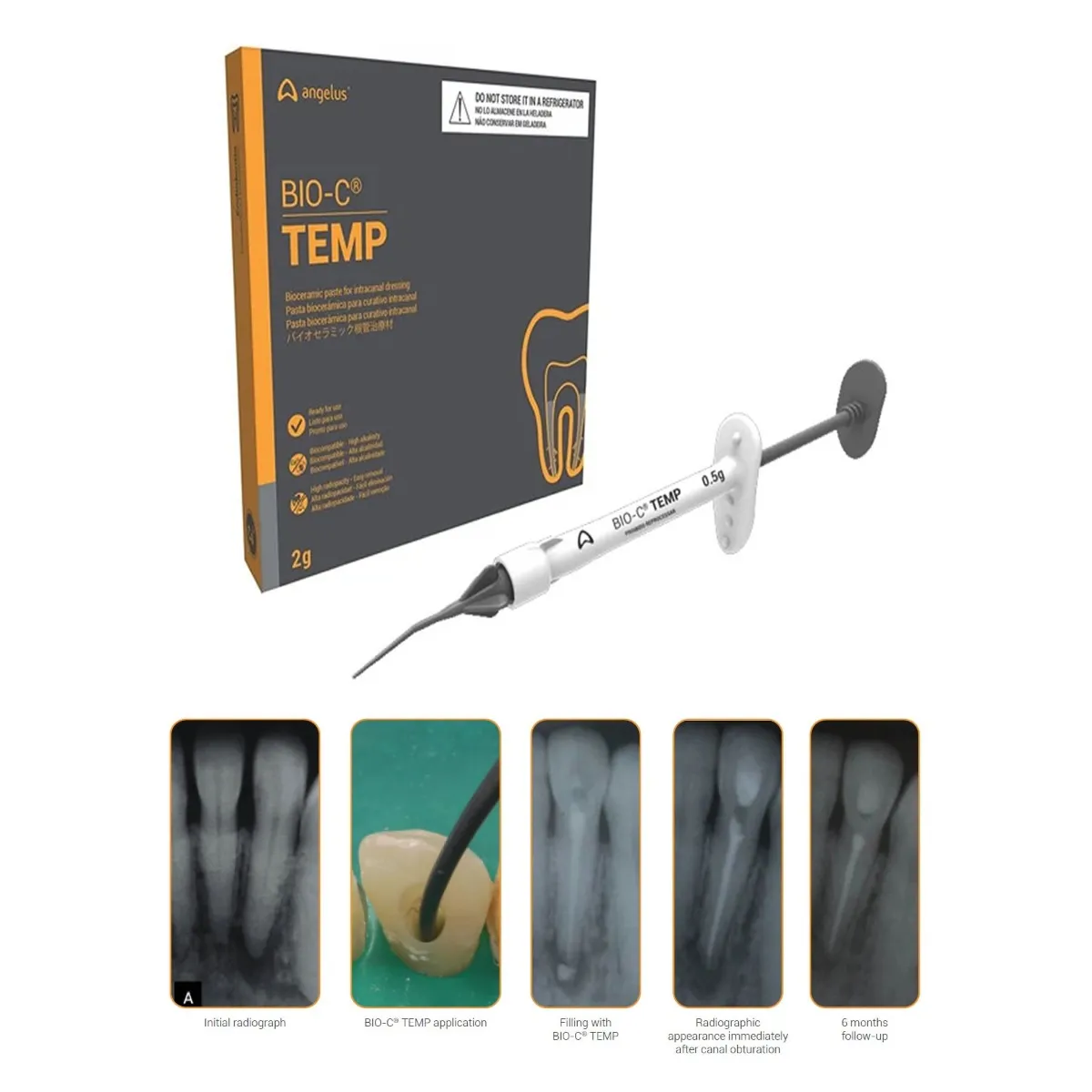
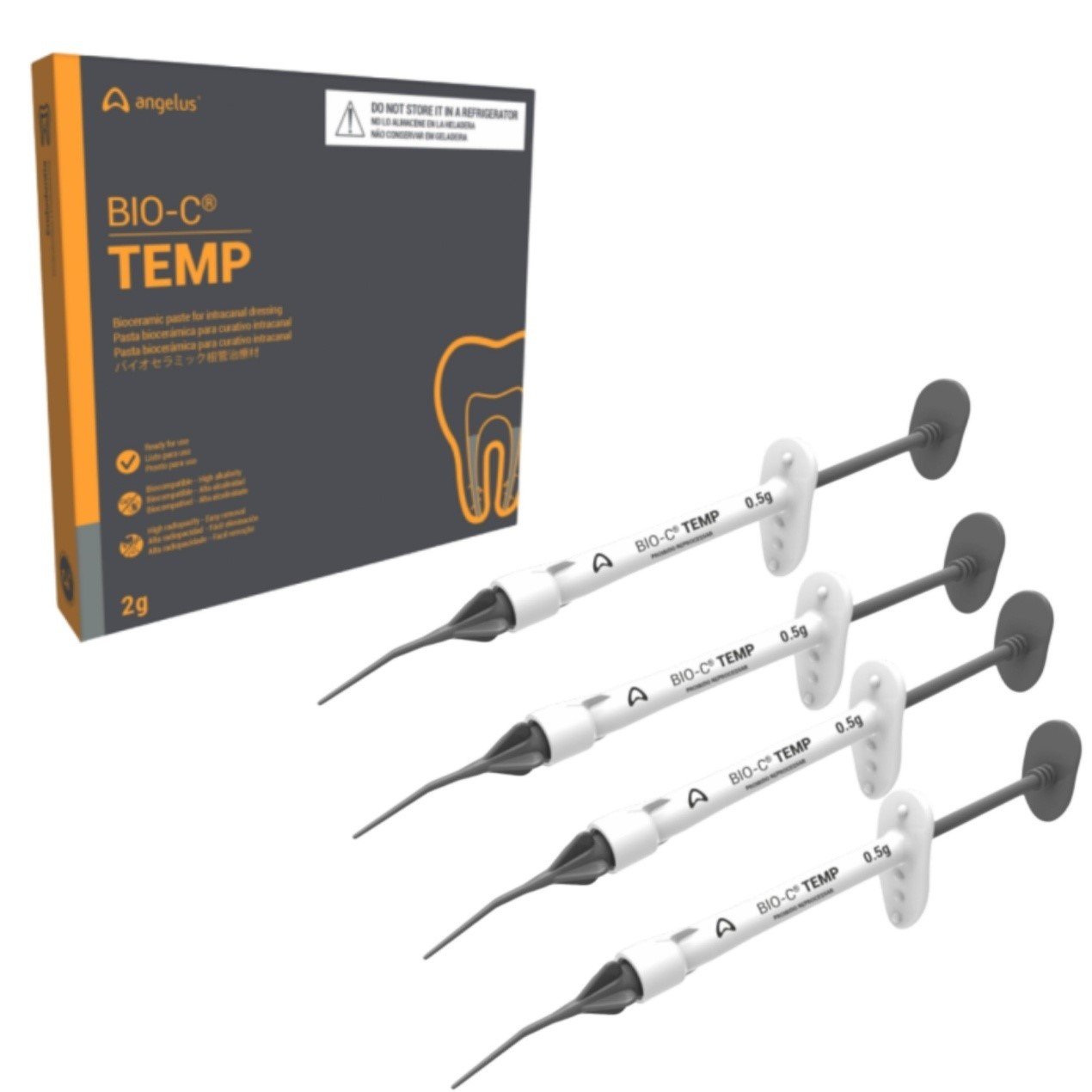
Product description
Meet BIO-C TEMP
BIO-C TEMP
- Bioceramic paste for intracanal dressing
Indication
- Intracanal dressing for endodontic Treatment of teeth with pulp necrosis and retreatment.
- Intracanal dressing for cases of perforations, external and internal resorptions, prior to the use of BIO-C REPAIR, MTA REPAIR HP and MTA ANGELUS®.
- Incomplete rhizogenesis.
- Trauma cases.
Advantages
- Gradual release of Ca2+: Does not require constant changes like calcium hydroxide;
- Biocompatible: Absence of postoperative pain due to the presence of salicylate
- High alkalinity: Eliminates bacteria due to high pH
- Easy to remove: Clinical time optimization;
- High radiopacity: Great radiographic visualization
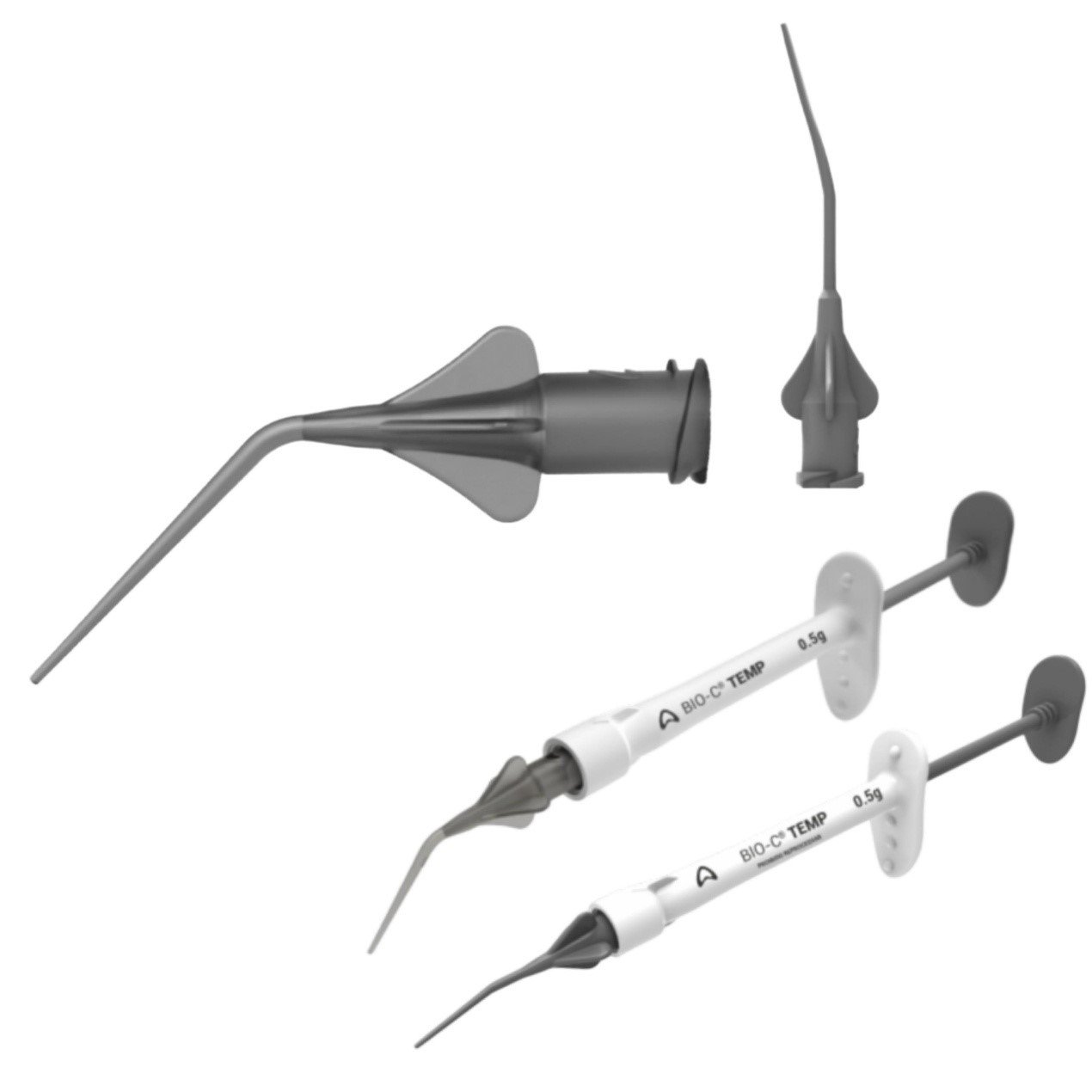
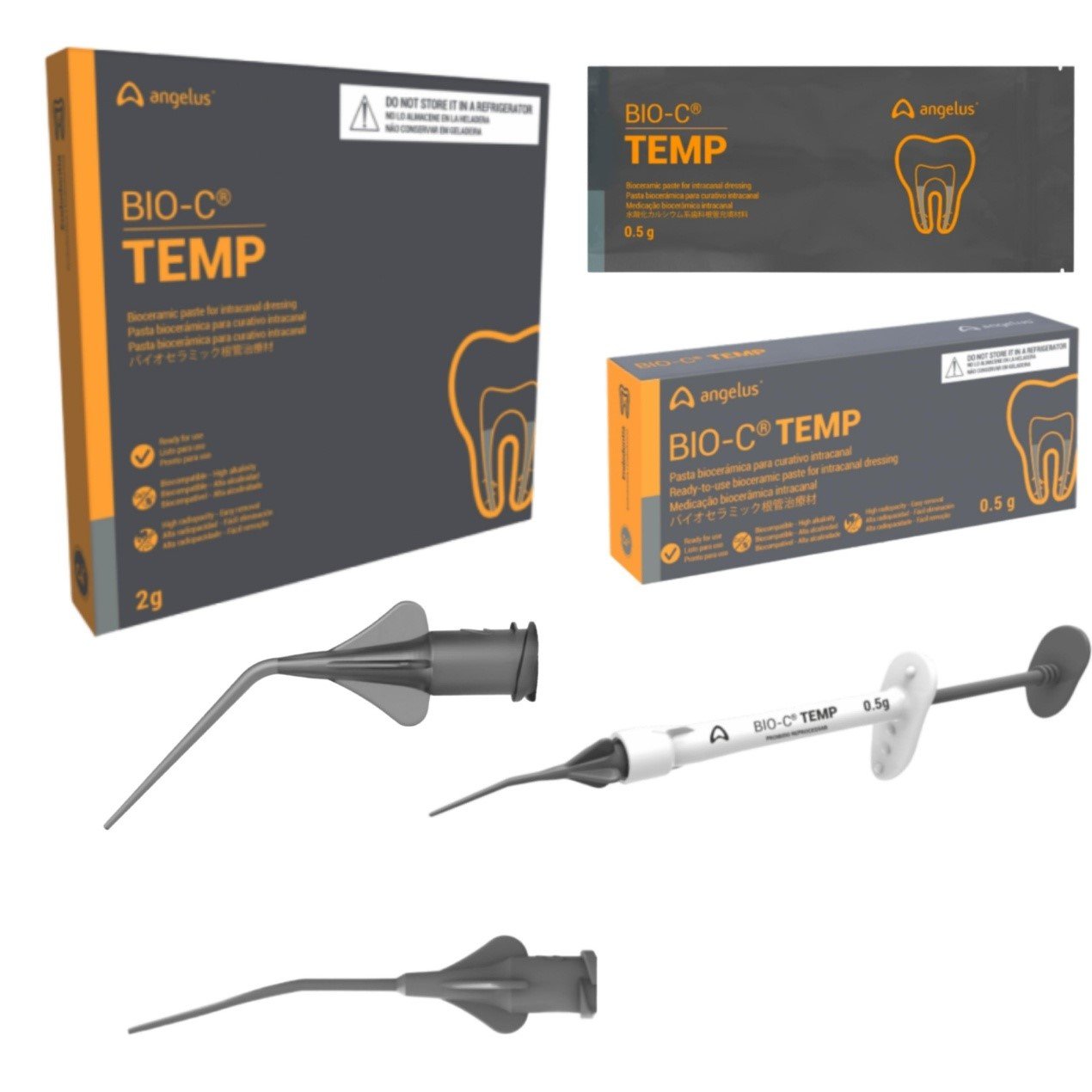
PRESENTATION
- Package with 1 syringe (0.5 g) and 5
- Application tips;
- Package with 4 syringes (0.5 g each) and 20 application tips.
Video about the product
Frequently asked questions(FAQ)
Answers to your questions and inquiries about the product
What is the difference between BIO-C® SEALER, BIO-C® REPAIR, and BIO-C® TEMP?
BIO-C® SEALER is a root canal sealer used primarily for obturation; BIO-C® REPAIR is a repair material; BIO-C® TEMP is a dressing interappointment intracanal temporary medication.
Does BIO-C® TEMP set inside the canal?
BIO-C® TEMP has properties by design that prevent it from setting in the canal allowing it to be used as an interappointment dressing material, being easy to be removed.
When to use the product?
In cases when instrumentation is unfinished, painful symptomatology, or presence of exudate.
How to perform the BIO-C® TEMP application?
Insert the cannula close to the apical third and apply BIO-C® TEMP gradually withdrawing the cannula to obtain complete filling of the canal(s). Use a temporary restoration material to promote coronal sealing.
How to remove the BIO-C® TEMP from inside the canal?
BIO-C® TEMP should be removed using Sodium Hypochlorite associated with the use of a K-type file, using brushing movements. After irrigate with 17% EDTA solution and, if possible, activate it with an ultrasonic tip in 3 cycles of 10 seconds duration.
How often should the medication be replaced?
BIO-C® TEMP provides a gradual and slow release of the components of its formula, aiming at apical tissue repair. Therefore, unlike calcium hydroxide that requires frequent replacements, BIO-C® TEMP disregards new applications. Persistent symptomatology should define re-evaluation of the procedure.
What is the antibacterial base of the product?
The main components of BIO-C® TEMP are Calcium Silicates, which, after being hydrated, produce Calcium Hydroxide that dissociates into Ca2+ and OH-. The released hydroxyl ions (OH-) are responsible for a significant increase in the pH of the surrounding tissue, which makes the environment inappropriate for bacterial growth.
What is the bactericidal effectiveness against the CaOH?
We have better pH maintenance results from BIO-C® TEMP over 30 days than Ultracal and Metapaste, which are based on calcium hydroxide. (Prof. Manoel Sousa-Neto, Project 4: Short and long-term monitoring of the intracanal ionic release capacity and pH promoted by BIO-C® TEMP. Compare statistically with CaOH2).
It has a direct relationship of effectiveness between higher pH values and a long time with antimicrobial activity.
The results of antimicrobial activity by direct and indirect contact presented in the attached article, inindicated that the antimicrobial activity of BIO-C® TEMP is similar to Ultracal
Brand or manufacturer
Meet the Medical Palace Partners

Anguls company
Founded in 1994, Angelus is a dental consumable manufacturer, located in the south of Brazil, specialized in the areas of Restorative Dentistry, Endodontics, Pediatric Dentistry and Laboratory.
Concentrating its core competence in R&D, with a distinguished team of PhD's and partnership with scientists, Angelus is able to generate a great number of patented innovative products.
The results of this background gave Angelus the National Innovation Award, considered the " Oscar " of Brazil innovation.
Angelus has 6 certifications, ISO 13485, EN ISO 13485 + AC:2007, Directive 93/42/CEE, ISO 9001, RDC 59 e FDA that guarantee the quality system and allows its worldwide distribution to more than 65 countries in the five continents. Focusing on fiber reinforced materials MTA-based solutions and a clever line of pediatrician products, the company sustains a two-digit growth sinceits foundation.
Angelus believes that Innovation is the drive force that brings growth and benefits not only to itself but also to the Dentistry field and the communities around the world.